A Prospective Randomised Open Label Study to Evaluate the Response Rate and Toxicity of Gefitinib in the Treatment of Carcinoma Cervix Patients on Chemoradiotherapy
DOI:
https://doi.org/10.5530/jcram.1.2.14Keywords:
Cervical cancer, Toxicity, Gefitinib, Cisplatin, KEY WORDS: cervical cancer, toxicity, gefitinib, cisplatin, EGFR inhibitorAbstract
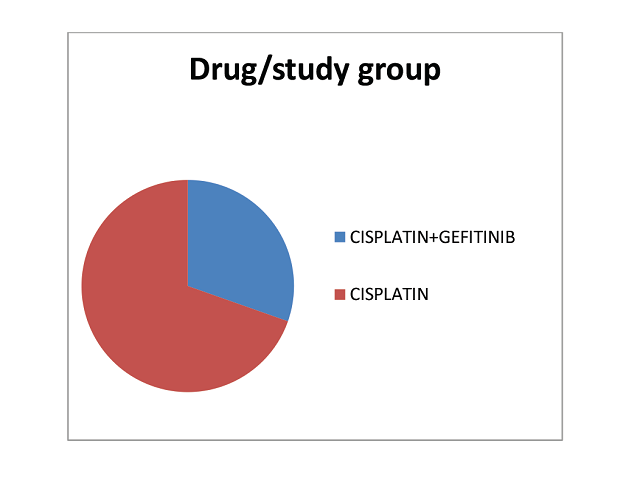
Introduction: Cervical cancer, on a global forum stands third most commonly diagnosed cancer. It is the fourth leading cause of cancer death in women. Despite with treatment of surgery, radiation and or chemotherapy, cervical cancer may persist or recur months or years after completion of initial treatment. Recently, the epidermal growth factor receptor (EGFR; HER1/erbB- 1) has been recognized as target for cancer therapy in numerous cancers. Clinical trials have demonstrated that only a subset of patients respond to EGFR inhibitors. Therefore the present study aimed to study the response rate of the drug gefitinib in patients receiving cisplatin-based chemotherapy in cervical cancer. Methods: Study was a randomized prospective study. Patients were randomized into two arms in the ratio 2:1 to get a statistically significant result. The sample size (n) was 16 in the experiment arm (Gefitinib+ Cisplatin) and 32 in the control (Cisplatin) arm. Toxicity and Response rate was assessed during the treatment, one month and 3 months post treatment. Results: A total of 23 subjects were included in the final analysis. On cisplatin were 16 (69.6%) and on Cisplatin+Gefitinib were 7 (30.4%). Our study attempted to evaluate the response rate and safety of gefitinib drug on locally advance cervical cancerous growth among 16 subjects, but because of the toxicity we couldn’t complete the study. Gefitinib was found to cause severe adverse drug reactions like grade 3 diarrhoea, fall in haemoglobin in many patients. Response rate was found to be satisfactory in both the arms. Conclusion: Due to overall small sample size, patients lost during treatment and unequal sample among the cisplatin and cisplatin +gefitinib group the analysis of disease-free period, adverse events could not be analysed. The efficacy of gefitinib among cervical patients was inconclusive and warrants further research in future.
Downloads
Published
How to Cite
Issue
Section
License
JCRAM and its contents are licensed under a Creative Commons Attribution-Non Commercial-No Derivs 4.0 License. Permissions beyond the scope of this license may be available with editor@jcramonline.com






